REVICE

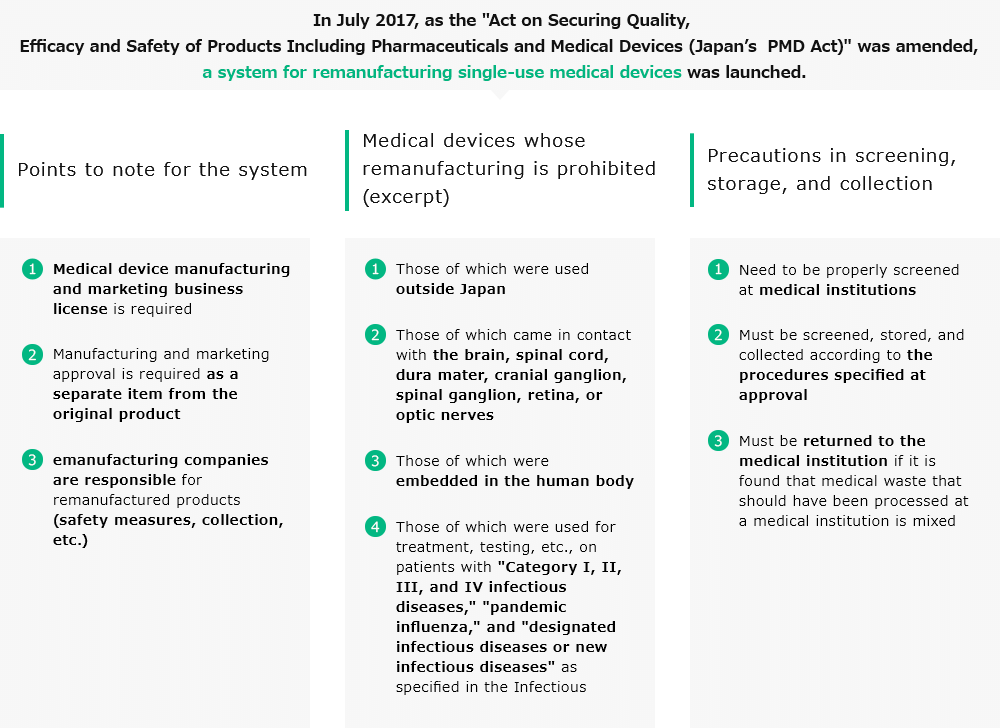
In July 2017, as the "Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical
Devices" was amended, a system for remanufacturing single-use medical devices was launched. Through remanufactured
single-use devices (R-SUDs), we will continue to protect medical safety and security to pass a sustainable society for
the future.
This is HOGY Medical's new challenge.
Introduction video on R-SUD business
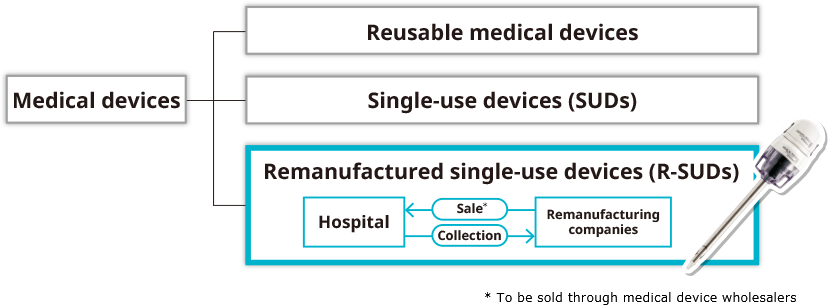
What is an R-SUD (remanufactured single-use device)?
Used single-use devices (SUDs) are collected from medical institutions by medical device manufacturers, and then checked, cleaned, sterilized, etc. (remanufactured) so that they can be reused as SUDs.
Source:
Ministry of Health, Labour and Welfare. Criteria for Remanufactured Single-Use Medical Devices (MHLW Ministerial
Announcement No. 261), July 31, 2017.
https://www.mhlw.go.jp/web/t_doc?dataId=00010630&dataType=0&pageNo=1 (Retrieved October 17, 2022)
Ministry of Health, Labour and Welfare. Amendment of the Enforcement Regulations of the Act on Securing Quality,
Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy
Products, and Cosmetics related to Remanufactured Single-Use Medical Devices (R-SUDs) (PSEHB Notification No. 0731-7),
July 31, 2017
https://www.mhlw.go.jp/web/t_doc?dataId=00tc2888&dataType=1&pageNo=1 (Retrieved October 17, 2022)
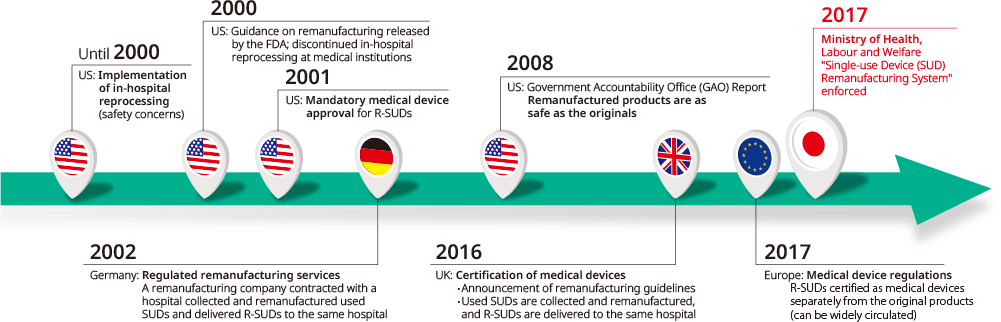
Current status of R-SUDs in Japan and other countries
While regulations and projects related to R-SUDs started around 2000 in other countries, Japan finally established a system and laws related to R-SUDs in 2017, and distribution began.
Health and Labor Sciences Special Research Project "Study on Remanufacturing of Single-Use Devices (SUDs), 2015
Comprehensive Research Report"
Prepared from https://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000170838.pdf(Retrieved
October 17, 2022)
What is expected from the spread of R-SUDs
Ensuring medical safety

Total inspection is fully performed on R-SUDs, which reduces defect rates.
Effective utilization of medical resources

By remanufacturing SUDs that are discarded after a single use, we contribute to the reduction of medical and welfare waste.
Provision of sustainable medical care
Manufacturing in Japan can expect to help address the risk of disruption in the global supply chain.
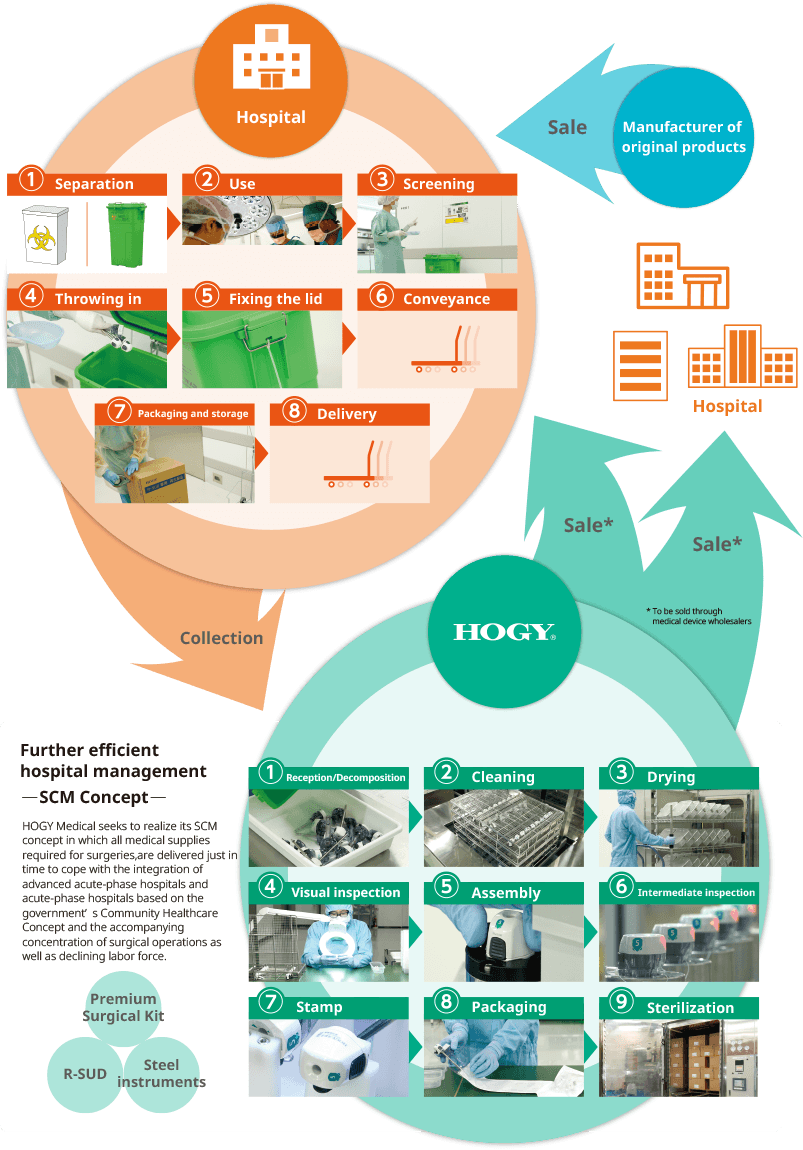
Idea of operation
REVICE product lineup
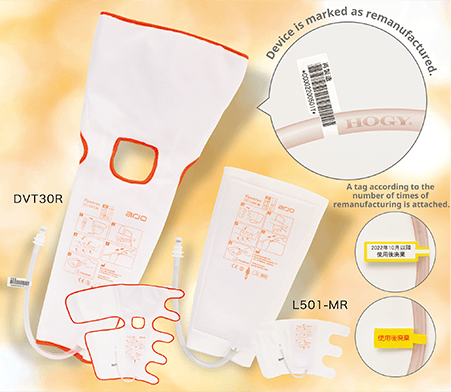
Remanufactured flowtron
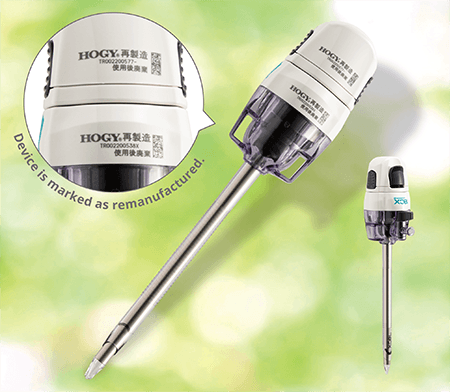
Remanufactured trocar
Environmental friendliness
Cleaning water generated during remanufacturing is purified by the equipment installed in our factory. We ensure that the quality of purified wastewater is high enough for "himedaka" (Japanese rice fish) to grow to conserve the environment.

