Plants & Facilities
Tsukuba Plant
A plant exclusively established for manufacturing our Premium Surgical Kits, for the creation of safe and high-quality products
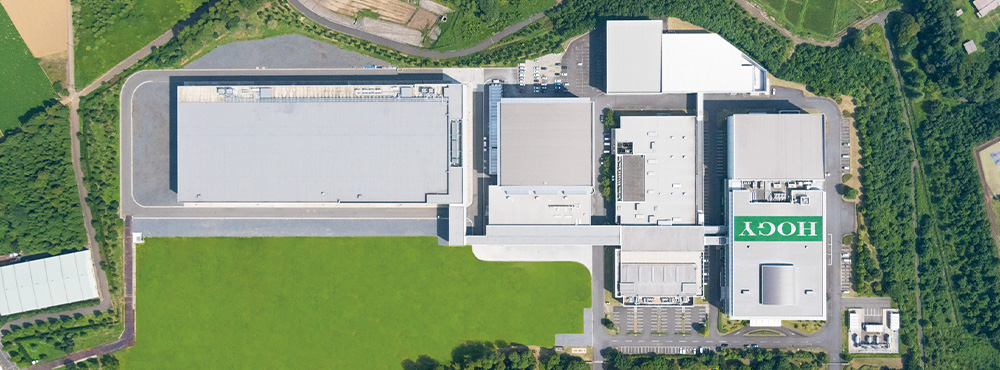
Realized integrated production and shipping lines
HOGY Medical has constructed a plant exclusively for its Premium Surgical Kits, aiming to achieve a stable supply of Premium Surgical Kits with higher safety and quality as well as shortened time required for delivery. Much of the plant is automated via advanced machines and robots, leveraging HOGY Medical's long-cultivated know-how. Equipped with functions both in terms of hardware and software, the plant safely and stably produces high-quality products that support the medical frontlines, which are in a stage of reform.

Improved safety through production by robots
Human-derived errors such as including incorrect items as well as the possibility of bacterial adhesion and foreign matter inclusion, which can occur in manual procedures, are reduced through production by advanced robots.

Seismic isolation structures employed at plant buildings
A seismic isolation structure employed for the building of the new plant and buildings is expected to withstand
earthquakes as strong as seismic intensity of 7 (on the Japanese seismic scale). Seismic isolation equipment is
installed underground in the seismic isolation structure, making the building less likely to be shaken by earthquakes.
As this function prevents robots from falling over, as well as other incidents, HOGY Medical expects that productive
conditions can be secured even in the period immediately following an earthquake.
Premium Surgical Kits
Click here for a detailed introduction to the Premium Surgical Kits manufactured at the new plant.
Tsukuba Sterilization Center

The Tsukuba Sterilization Center is equipped with safe and clean large-scale, electron-beam sterilization facilities. As the center is completely automated, it can be operated by a small number of staff and can conduct sterilization on large quantities of goods in a short period of time without removing packages.
Tsukuba Distribution Center

The Tsukuba Distribution Center is fully automated and conducts distribution processes precisely and speedily, enabling shipment of 10,000 units per day. Through this, HOGY Medical can respond to customer demand immediately.
HOGY Communication Path

HOGY Communication Path introduces the past, present, and future of HOGY Medical. It is this road that we have walked together with our customers since our foundation. You can learn more about HOGY Medical through an interactive experience that includes visual, touch, and sound inputs. You can experience HOGY from various angles such as through actual products, product catalogs, original publications, video contents, and brand-new graphic wall displays.
Facilities with Special Features
Electron-beam (EB) sterilization

Tsukuba Sterilization Center

Warehouse (Production Material)
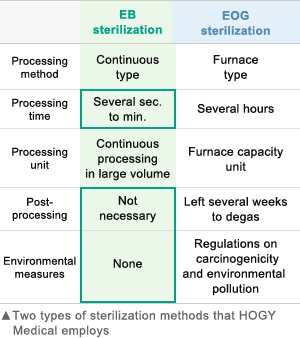
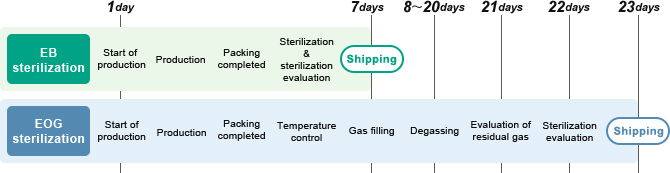
Currently, single-use medical devices are sterilized mainly through ethylene oxide gas (EOG) sterilization, electron beam (EB) sterilization, or gamma ray sterilization.
Hogy Medical uses EB sterilization for many of its products for the following reasons.
- Large quantities of products can be sterilized continuously and efficiently with short irradiation times (several seconds to few dozen seconds) while still in packages, allowing fast shipping
- EOG sterilization requires prolonged treatment before and after sterilization (such as heating and degassing the product), while EB sterilization requires no such treatment
- Gamma ray sterilization uses radioactive materials and EOG sterilization uses toxic gases, while EB sterilization poses little risk of environmental contamination

More than 30 years have passed since we became Japan's first surgical kit manufacturer to adopt EB sterilization.
In addition to complying with various regulations and laws, we set sterilization conditions for each product through in-house validation.We have also achieved full automation through computer control by combining our accumulated expertise in sterilization.
Other Plants

P.T. HOGY Indonesia No. 1 Plant
P.T. HOGY Indonesia, a subsidiary of HOGY Medical, is our East Asia production base. It is one of the world's leading medical-use non-woven fabric processing plants and is responsible for labor-intensive production processes.

Miho Plant No. 1

Miho Plant No. 2

Edosaki Distribution Center

P.T. HOGY Indonesia No. 2 Plant

HOGY Medical Co., Ltd. Miho Plant No. 2, Tsukuba Plant, and the Edosaki Sterilization Center were certified as ISO 13485:2016-compliant facilities.

Miho Plant No. 1 was certified as an ISO 9001:2015-compliant facility.

P.T. HOGY Indonesia No. 1 Plant and No. 2 Plant were certified as ISO 13485:2016-compliant facilities.
Head Office Showroom

A showroom at the head office highlights our entire product lines, providing a place for customers to learn more about our products. We believe this is a practical and effective way for improving communication and understanding to appropriately respond to customer needs.